ellume lab stock price
But last week the FDA gave an EUA to the first fully at-home diagnostic test that was made by an Australian company called Ellume. Australias largest stock trading and investment forum Australias 1 stock forum.
A Completely Nonscientific Guide To The Best Rapid Covid Tests
The price of the test is about 30 but Slavitt said that.

. Ellume has a global consumer health partnership with GlaxoSmithKline a global COVID-19 and latent TB partnership with QIAGEN and a range of professional products under its ellumelab brand. The test is called the Ellume. Company profile page for Ellumen Inc including stock price company news press releases executives board members and contact information.
As part of the contract Ellume is supplying the US. White House COVID-19 senior adviser Andy Slavitt said the Ellume test can detect COVID-19 with 95 accuracy within roughly 15 minutes. Ellume alos has a confidentially request the to FDA for detailed information their ellumelab device.
Ellume is committed to developing high-quality digital diagnostics. 06 2020 GLOBE NEWSWIRE -- Ellume the company reimagining digital diagnostics today announced a. GlobalNewsca your source for the latest news on ellume stock price.
Ellume has 198 employees across 2 locations and 23185 m in total funding. The San Diego California-based firm also announced the closing of its public offering of 7645259 shares of common stock at a price of 327 per share. Per a report by Grand View Research the global molecular diagnostics market was valued at 92 billion in 2019 and is expected to reach 182 billion by.
IT IS THE ONLY OTC ANTIGEN The Ellume COVID-19 Home Test is the only home test to use innovative fluorescent technology previously only used by doctors to detect COVID-19 infection. Historical data provides up to 10 years of daily historical stock prices and volumes for each stock. Ellume stock price videos and latest news articles.
This product has been authorized only for the detection of proteins from SARS-CoV-2 not for any other viruses or pathogens. The FDA has authorized more than 225 diagnostic tests for COVID-19 since the start of the pandemic including more than 25 tests that allow for home collection of samples which are then sent to a lab for testing. And the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the.
Dr Sean Parsons Ellumes founder and. Ellume has a global COVID-19 and TB partnership with QIAGEN and a range of professional products under its ellumelab brand. Government with 85 million of its home tests.
FREDERICK Md March 31 2022 PRNewswire -- Digital diagnostics company Ellume a producer of at-home COVID-19 test kits today officially opened its new 215000 square foot production. Both offerings generated combined proceeds of 1105 million which Progenity said it will use to support operations invest in molecular testing and precision medicine RD and for working capital and. The company will launch three rapid COVID-19 testing products in the USVALENCIA Calif Oct.
During the 2009 swine flu pandemic I was working as a physician in a busy emergency department. The Ellume COVID-19 Home Test has not been FDA cleared or approved. There will be a point the.
That can be bought at drug stores. The Company develops ellumelab a versatile and convenient diagnostic device that complements a doctors decision making. Anteobind incorporated in 10min Covid Test Antibody test in collaboration with Ellume Qiagen.
The Ellume -19 Home Test kit is an easy-to-use rapid antigen home test that detects an active -19 infection in 15 minutes without sending to a lab. This deal is set to boost the companys revenue. Scores of patients were presenting with symptoms of influenza.
White House coronavirus adviser Andy Slavitt says the government awarded a 231-million contract to scale up production of a COVID-19 home test recently authorized by US. See insights on Ellume including office locations competitors revenue financials executives subsidiaries and more at Craft. But has been authorized by FDA under an EUA.
Ellume was founded to address these problems through innovative approaches to diagnosis and treatment accessibility. Price Performance Shares of the company have gained 458 in the past year compared with the industry s 106 rise and SP 500s. Laboratory tests took several days to return results and the small.
Compound Annual Growth Rate CAGR Definition. Ellume has entered into a 232 million agreement with the US. Tuesday January 11 2022 Ellume Stock Price History Why Rapid At Home Covid 19 Tests Are Hard To Find.
As we continue to authorise additional Ellume tests for home use we are helping expand Americans access to testing reducing the burden on. The AFR tells us that Ellume chair and investor Paul Darrouzet holds a quarter of Ellumes shares. ANN The Ansell share price is down 14 to 2695.
The Ellume COVID-19 Home Test is the first COVID-19 test that can be used completely at home without a prescription.

Ellume Announces 231 8 Million Agreement With The U S Government Ellume

Ellume Covid Test A Rapid At Home Test New Low Price By Meenta
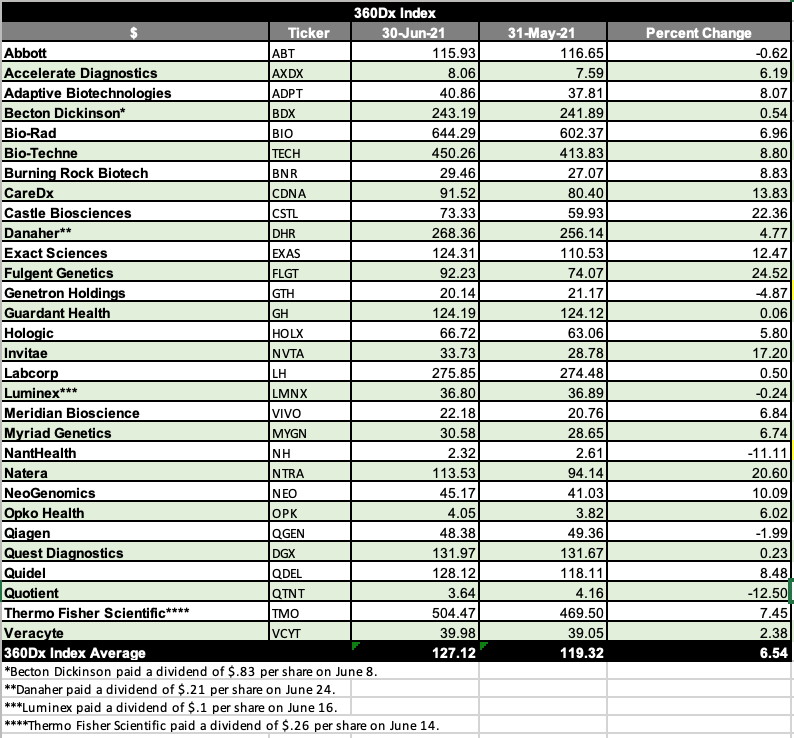
360dx Index Up 7 Percent In June Rebounding From May Downturn 360dx

Ellume Announces 231 8 Million Agreement With The U S Government To Scale Up Production Of Covid 19 Home Tests

Fda Ok S Over The Counter Home Test For Covid 19 Medcity News

Over The Counter Covid 19 Testing Now Available At Cvs Pharmacy Ellume

How To Shop For Fda Authorized Home Covid Test Kits A Guide

White House Will Spend Another 1 Billion On Rapid At Home Covid Tests Shots Health News Npr

Ellume Covid Test A Rapid At Home Test New Low Price By Meenta

Ellume Announces High Performance Test For Active Covid 19 Infection Ellume

Ellume Announces 231 8 Million Agreement With The U S Government To Scale Up Production Of Covid 19 Home Tests

Ellume Covid Test A Rapid At Home Test New Low Price By Meenta

Strong Start To 2018 For Diagnostics Companies Stocks 360dx

Ellume Announces 231 8 Million Agreement With The U S Government Ellume




